HEART TRANSPLANT: Man With Genetically Modified Pig Heart Dies, Medical Team Releases Findings
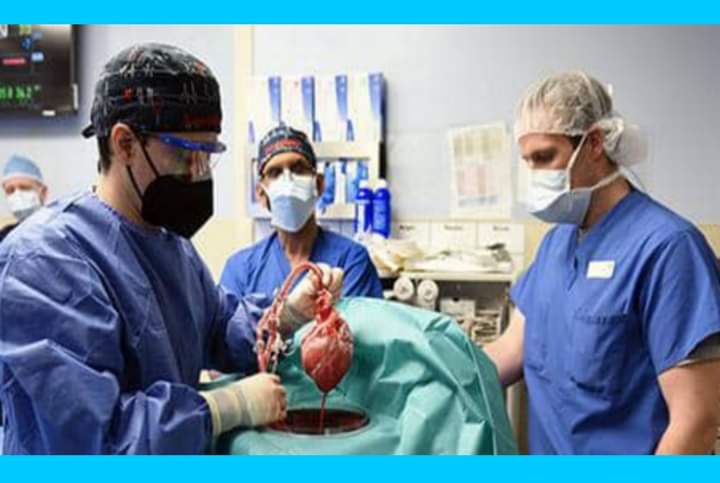
Did you know that David Bennett Sr., a 57-year-old man in the US was the first to receive a genetically modified pig heart in a first-of-its-kind transplant surgery at the University of Maryland Medicine?
David Bennett Sr. was diagnosed with a terminal heart disease known as arrhythmia, a condition where the heart beats with an irregular pattern. This condition led to him being hospitalised and bed-ridden for months, connected to a heart-lung bypass machine to remain alive. Much worse, after reviews of his medical records, he was deemed ineligible for a conventional heart transplant or an artificial heart pump, leaving him with only one option—a pig heart.
The US Food and Drug Administration granted emergency authorization for the genetically modified pig heart in a first-of-its-kind transplant surgery at the University of Maryland Medicine on Friday, 31 December 2021. A regenerative medicine company, Revivicor, based in Blacksburg, Virginia, US, had provided the pig heart.
In the process of the surgery, three genes that are responsible for rejection of pig organs by human immune systems were removed from the donor pig, and one gene was taken out to prevent excessive pig heart tissue growth. Six human genes responsible for immune acceptance were inserted.
According to surgeon Dr. Bartley P. Griffith, the director of the cardiac transplant programme at the medical centre, said, "There are simply not enough donor human hearts available to meet the long list of potential recipients. We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future."
Art Caplan, a professor of bioethics at US New York University, said the US has a "terrible" shortage of organs for transplants and believes engineering animal parts is a solution. However, "The question is, can we get there with minimal harm to the first volunteers?" he asked.
Before the historic surgery, Bennett Sr. had said, “It was either die or do this transplant. I want to live. I know it’s a shot in the dark, but it’s my last choice. I look forward to getting out of bed after I recover.”
Sadly, the patient Bennett Sr. who experienced strong cardiac function with no obvious signs of acute rejection for nearly seven weeks after the surgery, later had a sudden onset of heart failure that led to his death two months after the transplant.
The transplant team has been conducting extensive studies into the physiologic processes that led to the heart failure to identify factors that can be prevented in future transplants to improve the odds of longer-term success. A new study published on June 29, 2023, in The Lancet, revealed the most extensive analysis to date on what led to the eventual heart failure in the world's first successful transplant of the genetically modified pig heart into the patient. And what was their findings?
The new study confirms that no signs of acute rejection occurred during the first several weeks after the transplant. Likely, several overlapping factors led to heart failure in Mr. Bennett, including his poor state of health prior to the transplant that led him to become severely immunocompromised. This limited the use of an effective anti-rejection regimen used in preclinical studies for xenotransplantation. As a result, the researchers found, the patient was likely more vulnerable to rejection of the organ from antibodies made by the immune system. The researchers found indirect evidence of antibody-mediated rejection based on histology, immunohistochemical staining and single cell RNA analysis.
The use of an intravenous immunoglobulin, IVIG, a drug that contains antibodies, may also have contributed to damage of the heart muscle cells. It was given to the patient twice during the second month after the transplant to help prevent infection, likely also triggering an anti-pig immune response. The research team found evidence of immunoglobulin antibodies targeting the pig vascular endothelium layer of the heart.
Lastly, the new study investigated the presence of a latent virus, called porcine cytomegalovirus (PCMV), in the pig heart, which may have contributed to the dysfunction of the transplant. Activation of the virus may have occurred after the patient's anti-viral treatment regimen was reduced to address other health issues. This may have initiated an inflammatory response causing cell damage. However, there is no evidence that the virus infected the patient or spread to organs beyond the heart. Improved PCMV testing protocols have been developed for sensitive detection and exclusion of latent viruses for future xenotransplants.
Pig heart valves have been transplanted into humans for many years. In October 2022, surgeons successfully tested the transplant of a genetically modified pig kidney into a woman in New York who was brain-dead. More than 40,000 transplants – a record – were done in 2021, according to the Organ Procurement and Transplantation Network.
"Valuable lessons can be learned from this groundbreaking surgery and the courageous first patient, Mr. Bennett, that will better inform us for future xenotransplants," said UMSOM Dean Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. "In the future, our team of surgeon-scientists will utilize newly designed immune cell assays to monitor the patient more precisely in the days, weeks, and months following the xenotransplant. This will provide stricter control of the earliest signs of rejection and the promise of a truly lifesaving innovation."
Sources: CNN | Medical Express
#penglobalhealth